A new study published in the journal eLife asked a very simple question: What cell and tissue-level changes occur in the testes of someone receiving feminizing hormone therapy?
Admittedly, this question feels handcrafted for the cis gaze with its fixation on the genitals of trans people (especially transfemmes). Yet, the decontextualized scientific question remains interesting even if it requires rephrasing: How does estradiol affect the cells and tissues of the adult testes? Certainly, this question reveals itself in a transsexual context, but research thus far has focussed only on testosterone’s role in testes development. As a result, we still don’t know how estrogen influences the testes.
Nevertheless, the question is fascinating because the testes has two major functions: to produce sperm (in a process called spermatogenesis) and synthesize androgens. As such, the testes are very sensitive to sex hormones, and spermatogenesis requires testosterone. During feminizing hormonal transition, the testes first slow and then (often) halt spermatogenesis. This is why doctors recommend fertility preservation when starting feminizing hormones.
Sperm-generating region of the adult testes is called a seminiferous tubule which has a characteristic morphology: a hollow tunnel of cells with mature sperm in the center and intermediate stages of cellular maturity along the edges. The edges are also home to a specialized cell type called Sertoli cells. Sertoli cells provide structure to cellular tunnel and produce hormones that ensure efficient sperm production (a process called spermatogenesis).
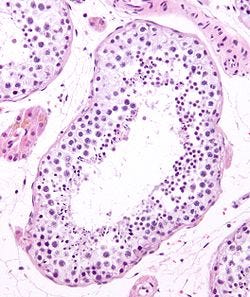
In contrast, an immature testis lacks seminiferous tubules and their precise organization. Instead of a hollow tunnel, the cells exist as an amorphous mass. Further, the cells themselves are immature. For example, instead of Sertoli cells you can see the precursors to Sertoli cells. These precursors are examples of stem cells, meaning that they can clone themselves through cell division and that they are still capable of further specializing their cell identity. Puberty induces the cells to specialize (in a process called differentiation) and reorganize into the cellular architecture seen in adults.
The Belgian research team of the eLife study analyzed the testes of 109 transfeminine people who were receiving hormone replacement therapy (HRT) of estradiol and a testosterone blocker (cyproterone acetate). The researchers acquired the tissue following the participants’ bottom surgeries, and all subjects consented to the donation. The cis participants also granted their consent to donate tissue as part of a necessary medical procedure such as a biopsy or orchiectomy.
When examining testes from transsexual donors, the authors immediately noticed that the seminiferous tubule structure was absent. Instead, these testes showed an unorganized cell pattern seen in tissues from prepubertal donors. They confirmed this by looking for the presence of proteins that signify mature and immature Sertoli cells. These images are shown below, where Sox9 (yellow) is found broadly in testes, androgen receptor (AR, red) is found in mature Sertoli cells, and anti-Müllerian hormone (AMH, green) is found in Sertoli cell progenitors.
(Note: In adult and peripubertal tissues, AMH is normally found outside of the seminiferous tubules such that it creates the image pattern shown below. Sertoli cells are shown in yellow which does not overlap with the green AMH signal.)

From this analysis, transsexual testes appear more similar to younger testes than adult, cis testes. However, trans samples had some unique features. The research team was interested in getting a more “zoomed out” view of the gene profiles in the testes samples. To do this, they performed RNA sequencing which reports back the relative quantities in which genes are expressed at the mRNA level in a sample. The resulting data was then analyzed to identify genes (called differently expressed genes, or DEGs) that showed coordinated changes between the four donor groups.
This analysis (shown below) groups each of the 11,661 DEGs as a single row, grouped by individual and with genes which show similar trends across donor identities. The grayscale color indicates the relative amount of that gene found in a particular donor. At the mRNA level, the testes of transfemmes on HRT bear some resemblance to younger testes. However, there is a unique group of genes that are only expressed in the transsexual samples (the rows marked with the red bar on the left). These genes are associated with immune system activity.1 This means that the transsexual testes a unique cell state with some reactivation of some developmental pathways.
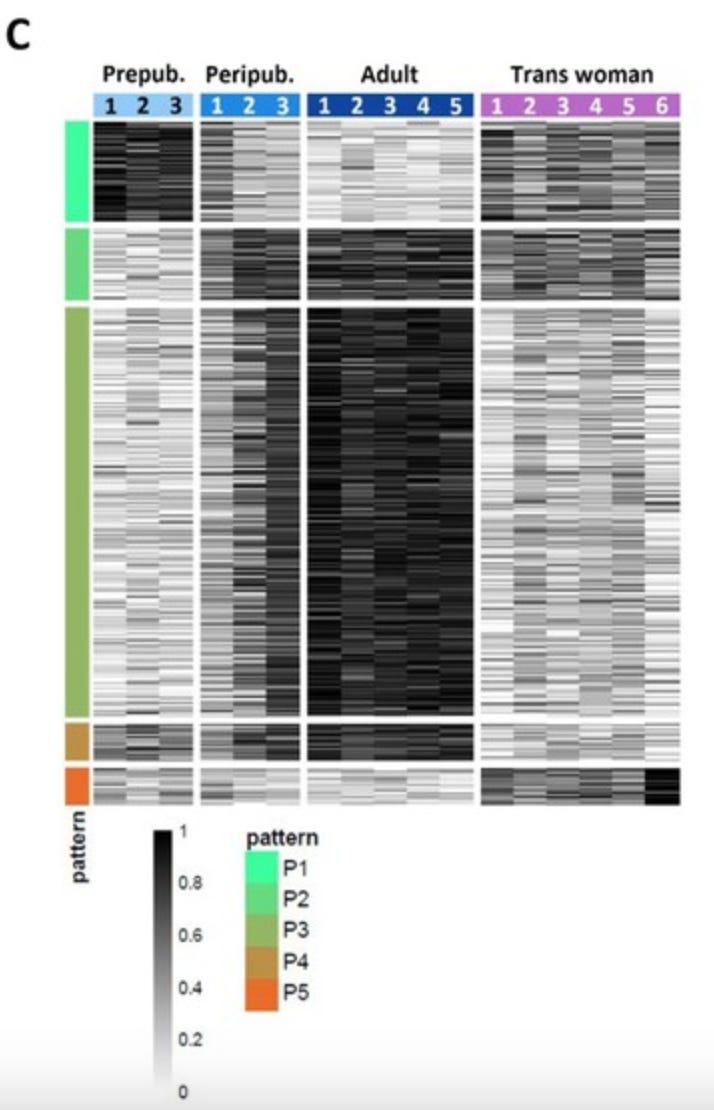
Together, these results are consistent with decreased fertility for transfemmes upon starting HRT. They also suggest that if HRT is stopped for any reason that spermatogenesis would resume by shifting cells back toward a mature state as testosterone rises. Indeed, this is consistent with a report from 2023 that found sperm production does resume in people who stop taking feminizing hormones. Estrogens are not permanent sterilizing agents for people who produce sperm.
At heart (and by training), I am a developmental2 biologist, so I hope that you will indulge me as I offer my hypothesis on how sex hormones are functioning in the postnatal testes. Testicular cells are likely responding to the balance3 between androgens and estrogens. Before puberty, low testosterone levels (and therefore relatively higher estradiol levels) likely maintain the cells as progenitors which are not yet capable of making sperm. When testosterone spikes during puberty, these cells differentiate and spermatogenesis begins.
When estrogen levels increase over a long period of time (could be for various reasons, including starting feminizing hormone therapy), then the testes interprets this new hormonal balance to de-differentiate and become stem cells once again. To get rid of the specialized sites of spermatogenesis, they signal to the immune system to break them down. This strategy is very similar to how the brain gets rid of unwanted neural connections during forgetting and “unlearning.”
Of course, this is just my hypothesis. One way we could test this is to perform this analysis on the testes of older cis men. Since testosterone levels decline with age, the androgen-estrogen balance also decreases with age. If the testes were interpreting hormonal balance, then the histological and RNA-level analyses may show similar results to those of transfeminine people on HRT.
But first we are going to need some consenting tissue donors ;;; and a lot of money in research funds.
Doctors aren't trained in queer health. But you can help!
If you are a queer person, you probably have had at least one bad experience with the medical system. Maybe a doctor asked you invasive questions about your sexuality or sexual practices. Or, they misgendered you (even after you shared your pronouns). Perhaps they didn’t know what you were talking about when you asked a…
Immune system activity is not necessarily bad. More on this in a bit…
Personally, I consider development to encompass any process by which bodyminds change over time, including aging.
This is a relative balance given that all people (regardless of sex assigned at birth) have higher androgen levels than estrogen levels.







Does this imply that once in this prepubescent undifferentiated stem cells like state, that it could be possible we could coax differentiation into granulosa cells instead of sertoli cells?
TIL stem cells are stored in the orchis!